PRAKTYCZNE ASPEKTY STOSOWANIA INSTILAN – NOWOCZESNEGO PROTEKTORA BŁONY ŚLUZOWEJ PĘCHERZA MOCZOWEGO
E.O. Stakhovsky, PhD, MD, Professor, Head of the Scientific and Research Department of Plastic and Reconstructive Oncourology, M.V. Chepurnaty, SI "National Cancer Institute" Ministry of Health of Ukraine, Kyiv
Powierzchnia wewnętrzna pęcherza moczowego pokryta warstwą glikozoaminoglikanową (warstwą GAG). Podstawową funkcją tej warstwy jest tworzenie cienkiego filmu ochronnego poprzez wiązanie wody.
The inner surface of the urinary bladder is covered by glycosaminoglycan layer (GAG-layer). The main task of this layer is to bind water, forming a thin protective film between urine and the surface of the urinary bladder. This protective film prevents bacteria and other irritants, contained in the urine (e.g., potassium ions, calcium microcrystals, protease), from affecting the urinary bladder that can cause inflammation and pain [1].
It has been proved that the GAG-layer simultaneously performs several important functions: it protects the deeper layers of the urinary bladder walls from irritants’ penetration from the urine; prevents giving rise and increase of inflammation, as well as reduces the degree of pain caused by inflammation [1, 21].
Urothelium defects are the cause of many chronic diseases of the urinary tract (interstitial cystitis, recurrent bacterial cystitis, irradiation cystitis, overactive urinary bladder, etc.), accompanied by the appearance of such symptoms as urgent and frequent urination, urinary incontinence, pain during urination, syndrome of urinary bladder chronic pain. Such patients are largely limited in their daily activities and suffer from reduced quality of their life [2, 3].
The mechanism of pain in the bladder:
- Damage of GAG-layer.
- The destruction of the epithelial layer by aggressive structures.
- Inflammation in sub-epithelial structures.
- Activation of C-fibres and release of Neuropeptides.
- Neurogenic inflammation.
- Progression of fibrosis, scarring, shrinkage of the urinary bladder.
Chronic inflammation of the urinary bladder can lead to the replacement of its muscular wall by fibrous tissue, as well as sclerosis and ultimately to the development of microcysts [21]. To solve this problem, the therapy designed to restore the protective layer of urothelium is recommended.
Today in clinical practice different treatment strategies are used. They suggest prescription of antidepressants, antihistamines, cysteine, leukotrienes receptors antagonists, internal bladder instillation of dimethyl sulfoxide to patient, but when the pain syndrome is present, opioid analgesics are prescribed. In difficult cases surgery can be performed (augmentation of urinary bladder, cystectomy with application of urine derivation methods), which is the only method of solving this problem [13-15].
In recent years the application of therapy that helps to restore the GAG-layer is actively studied.
The most studied among glycosaminoglycans are hyaluronic acid, chondroitin sulfates, dermatan sulfates, keratan sulfates and heparan sulfates that compose skin, tendons, cartilage, joints, providing mechanical strength and elasticity of inner organs, as well as elasticity of their joining [12].
In particular, hyaluronic acid is a glycosaminoglycan which, according to its chemical nature, is a branched chain of repeated disaccharide components (Fig. 1). At pH 7.0 level carboxyl groups of hyaluronic acid are fully ionized, bearing a negative charge and interacting with water molecules to form jelly-like matrix [12].
The name “Hyaluronic acid” was first suggested in 1934 by K. Meyer and J. W. Palmer, who identified this substance of the vitreous body of the eye. Its name comes from the Greek word hyalos – “glassy” and “uronic acid” [11].
It has been proved that glucosamine, chondroitin and hyaluronic acid have anti-inflammatory and analgesic effects, as well as water-containing biopolymer with silver ions has antibacterial effect, and the hyaluronic acid has derma-protective effect.
| Table 1. Clinical Research of Hyaluronic Acid in Urology | ||||
| Research | No of patients | Diagnosis | Prescription | Result |
| Miodosky et al. (2006) [8] | 7 | Hemorrhagic cystitis | 4 weekly injections + a monthly dose | Among 6 out of 7 patients – satisfactory result |
| Daha et al. (2005) [16] | 48 | Interstitial cystitis | 10 weekly injections | Improvement was observed in 41 case |
| Gupta et al. (2005) [17] | 36 | Interstitial cystitis | 6 weekly injections | 20 patients had a positive response for treatment |
| Kallestrup et al. (2005) [18] | 20 | Interstitial cystitis | 4 weekly injections
+ 2 monthly doses |
Among 40% of patients nuctoria symptoms decreased, among 30% – pain intensity decreased.
Thus, 13 patients showed positive result of this treatment |
| Constantinides et al. (2004) [19] | 40 | Recurrent inflammatory manifestations of the lower urinary tract | 4 weekly injections
+ 2 monthly doses |
During 5 months – no relapses among 40 patients, during up to 2 months – among 28 patients |
| Leppilahti et al. (2002) [20] | 11 | Interstitial cystitis | 4 weekly injections | During 1 year 8 patients reported positive result |
| Morales et al. (1997) [8] | 25 | Interstitial cystitis | 4 weekly injections + a monthly dose during 1 year | 71% patients showed positive response for treatment |
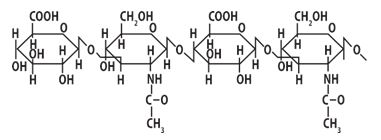
Fig. 1. Fragment of Hyaluronic acid structure
 |
– Instylan group
– Control group |
||||
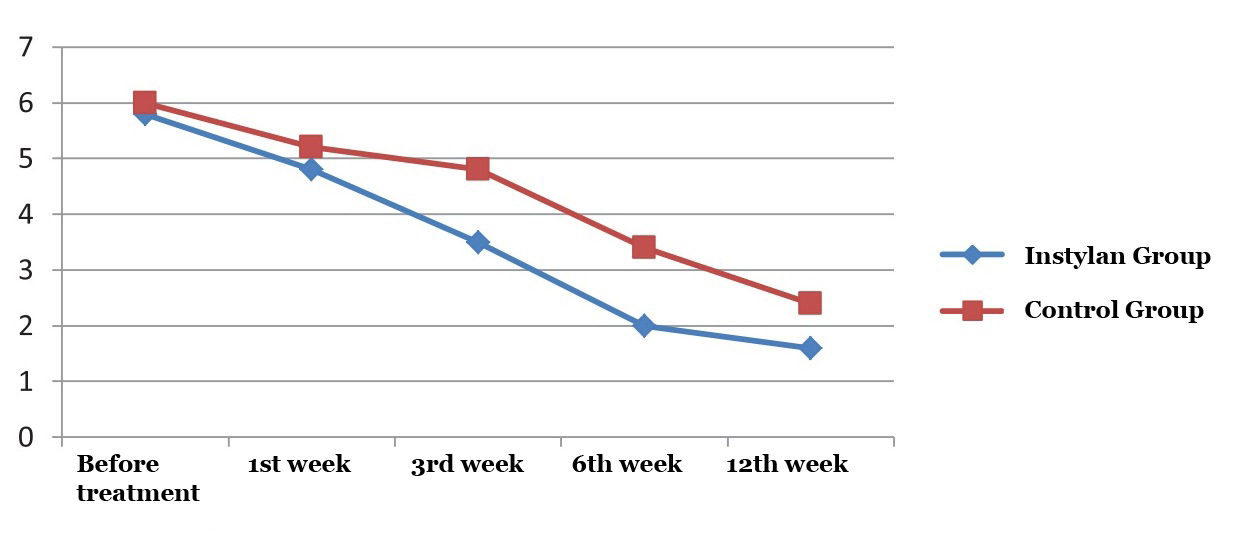
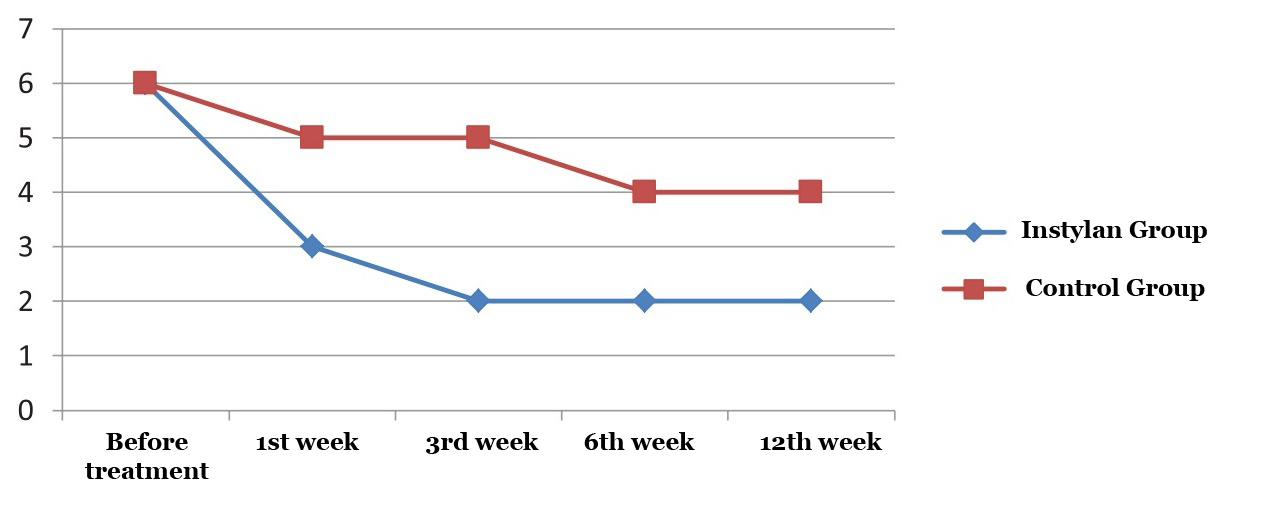
| Before treatment | 1st week | 3rd week | 6th week | 12th week | Fig. 2. The average frequency of nocturia in studied groups |
 |
– Instylan group
– Control group |
||||
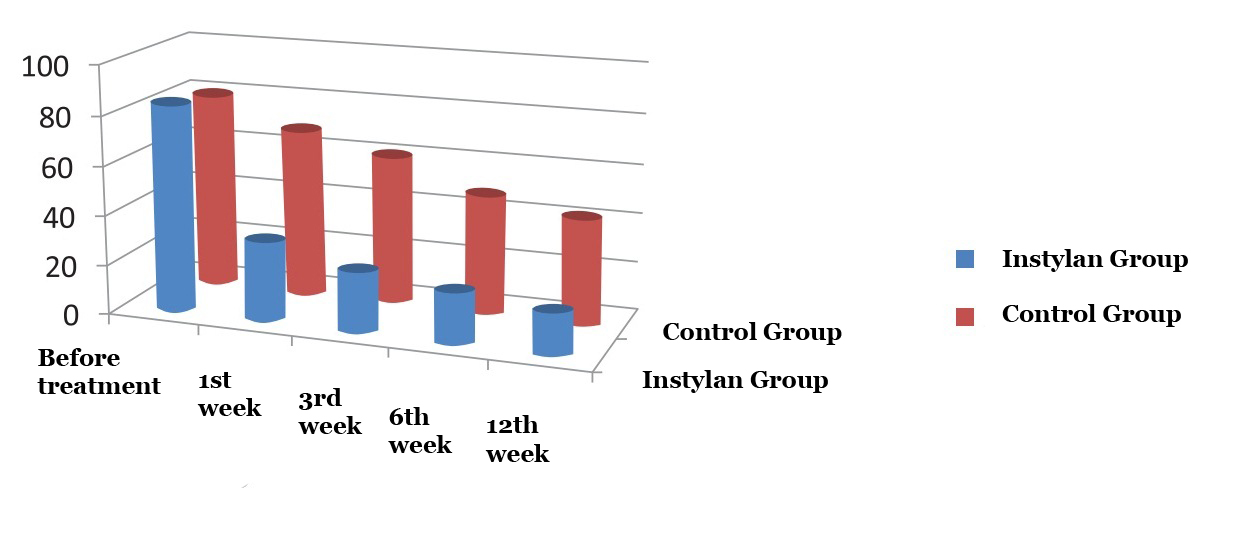
| Before treatment | 1st week | 3rd week | 6th week | 12th week | Fig. 3. Comparative assessment aimed at determination of residual urine |
 |
– Instylan group
– Control group |
||||
| Before treatment | 1st week | 3rd week | 6th week | 12th week | Fig. 4. The index of quality of life (L) |
Ulia\D:/2015/Urologija_Uria_Farm_Instilan_urologija
| Table 2. Structure of the disease among studied | |
| Diagnosis | No of cases, n (%) |
| Irradiation cystitis | 16 (43%) |
| Bladder cancer | 15 (32%) |
| Interstitial cystitis | 6 (16%) |
The Use of Hyaluronic Acid in Medicine
Given that hyaluronic acid comprises many tissues (skin, cartilage, vitreous body of the eye), it is used for curing diseases connected with these tissues: in ophthalmology (cataract treatment) [5] orthopedics (osteoarthritis, osteochondrosis, spondylosis, periarthritis) [4], cosmetology and cosmetic surgery (as intradermal injection) [6] stomatology (treatment of gingivitis), pulmonology (asthma treatment) [7], urology (treatment of cystitis, uric ureter reflux) [8-10].
Clinical research of intra cystic application of hyaluronic acid injections [22] is presented in Table 1. In order to assess the effectiveness of 50 mg / 80 mg sodium hyaluronate (Instylan, “Uria Farm”) intra cystic use in the treatment of inflammatory changes of the lower urinary tract, the authors of this article conducted the research, based on the Department of Plastic and Reconstructive Oncourology, SI “National Cancer Institute” from March to June 2015. The research involved 37 patients, 18 of whom were treated with the help of intra cystic use of 80 mg sodium hyaluronate (Instylan). The control group consisted of 19 patients who underwent basic antibacterial and anti-inflammatory therapy. The duration of observation was 3 months. First medical examination of patients coincided with the day of the treatment start, and assessment of treatment efficiency was done during the following visits (2-8).
Intra cystic intake of Instylan (50 mL of 0.16% sodium hyaluronate solution) was carried out according to the instructions once a week for 4 – 6 weeks with its further intake once a month. Installation was performed with the help of urethral catheter in an ambulatory care clinic with observance of aseptic rules.
The study evaluated the dynamics of clinical symptoms, complaints about frequent, painful urination, urgent urination, urge incontinence, nocturia; uroflowmetry indices, residual urine volume, quality of life.
The study involved 20 (54%) men and 17 (46%) women, whose average age was 42.5 years old. The research group consisted of the highest number of patients with irradiation cystitis – 43% (Table. 2).
The division of patients was based on pathological conditions that were treated with the help of intra cystic intake of Instylan. Concerning irradiation cystitis, 6 patients were treated with 6 weekly intra cystic injections; 9 patients took only one Instylan injection after removal of urethral catheter in case of the bladder wall transurethral resection (TUR); 3 patients had intra cystic injections every 4 weeks with regard of treating interstitial cystitis (Table. 3).
The average frequency of urge incontinence per day among patients who took Instylan decreased from 12.4 to 7.8 times comparing to the control group: from 12.8 to 10.4 times a day during 3 months of the study.
The most pronounced decrease of dysuric manifestations occurred within 1-6 weeks of treatment (Table. 4). In particular, 9 patients who took only one Instylan injection after removal of urethral catheter, reported a significant reduction in the frequency of urge incontinence from 10.2 to 4.8 times compared to the control group: from 9.8 to 6.6 times during the first week after instillation.
By 12 weeks of the study the frequency of nocturia in patients who applied Intra cystic intake of Instylan decreased by 72.4% (from 5.8 to 1.6 times) compared to the control group of patients – 60% (from 6 to 2.4) (Figure 2).
According to uroflowmetry indices, maximum and average speed of urination in both study groups did not differ significantly, but the average volume of urine in the control group of patients increased by 88 ml in the group of patients that took Instylan – 138 ml. Moreover, in the Instylan group the urination act volume increased sharply during the first 3 weeks of treatment (Table. 5).
Given the research data, in the period of up to 12 weeks of observation the average volume of residual urine decreased in both groups: 16 ml – in the studied group of patients, to 42 ml – in control group. It is particularly worth noting that patients who took Instylan reported that after the first week of treatment average volume of residual urine reduced from 84 to 32 ml (more than 2.5 times) compared to the control group – b from 80 to 68 ml (Fig. 3).
After 3 weeks of the study passed 16 (83%) patients, who were treatment with the help of Instylan, rated satisfactory quality of life (quality of life index L = 2) compared to the control group (index of quality of life L = 4), which in its highest position did not achieve satisfactory level until the end of research (Fig. 4).
Thus, intra cystic use intra cystic application of sodium hyaluronate (Instylan) is an effective treatment for various forms of chronic cystitis in the early postoperative period after transurethral resection of the bladder wall. Such result is achieved by reducing the effects of dysuria, nocturia frequency, residual urine volume, increased volume of urination that ultimately provides significant increase of patients’ quality of life.
References can be found in editorial office
Poster:
| Instylan – sterile solution on the basis of hyaluronic acid for intravesical intake |
| Natural restoration of urinary bladder barrier function |
| State of destroyed layer / Partially restored / fully restored layer |
| Restores and protects the glycosaminoglycan layer of urinary bladder mucosa;
accelerates the regeneration of the destroyed urothelium; reduces chafing of urinary bladder and prevents pain syndrome; contributes improvement of urination. |
| Instruction for medical use of medical product “Instylan” – sterile solution on the basis of hyaluronic acid for intravesical intake. Components: sodium hyaluronate – 80 mg; Phosphate buffer – pH 7,3 – up to 50 ml; indications for use: the solution is designed fot temporary protection and restoration of urinary bladder mucosa during different manipulations (urétrocystoscopie, radiotherapy etc.) Chronic / recurrent cystitis, Irradiation cystitis, Interstitial cystitis, hyperactive urinary bladder, urinary retention or tumor formation, caused by cystitis. Contraindication: Hypersensitivity to any of the components, prohibited for pregnant or breastfeeding women, as well as children. Application mode and doses: Instylan is designed for installation in urinary bladder conducted by specially trained doctor in the specially equipped room with observance of aseptic rules. Instylan is designed for intra cystic intake once a week. Treatment may include from 4 to 12 installations. The temperature of package should be no less than 20°C before its installation. Urinary bladder should be empty before Instylan installation is conducted. Instylan is input in urinary bladder cavity with the help of urological catheter for the period from 30 minutes to 2 hours. Registration No. of Ministry of Health of Ukraine 13660/2014 from 16.10.2014. |
Przed rozpoczęciem stosowania leków oferowanych na tej stronie należy skonsultować się z lekarzem a także dokładnie przeczytać ulotkę.
Informacje o wyrobie medycznym przeznaczone są dla pracowników ochrony zdrowia. INSTYLAN 0,16% jest bezbarwnym, przezroczystym lepkim sterylnym żelem kwasu hialuronowego pochodzenia niezwierzęcego o fizjologicznym PH.
INSTYLAN 0,16% został zatwierdzony jako wyrób medyczny IIа klasy. Deklaracja zgodności CE. Numer rejestracyjny: DD 60107286 0001. Wytwórca: Diaco Biofarmaceutici S.R.L. Via Flavia 124, 34147, Trieste, Italy.