Intravesical instillation of high molecular weight sodium hyaluronate in radiation‑induced cystitis: a prospective pilot study
Michael Baboudjian, Department of Urology and Kidney Transplantation, La Conception Hospital, Aix-Marseille University, APHM, Marseille, France
Marc Fourmarier, Department of Urology, CH Aix-Pertuis, Aix-en-Provence, France
Christophe Clement, Department of Urology, Clinique Rhône Durance, Avignon, France
Arnaud Cherasse, Department of Urology, Clinique du Val d’Ouest, Ecully, France
Jean‑Pierre Graziana, Department of Urology, Clinique Mutualiste de la Porte de L’Orient, Lorient, France
Youssef Bentaleb, Department of Urology, CH Jean Rougier, Cahors, France
Yohann Rouscoff, Department of Urology, Clinique Saint George, Nice, France
Sylvain Ducrocq, Department of Urology, CH Notre Dame de la Miséricorde, Ajaccio, France.
Bastien Gondran‑Tellier, Department of Urology and Kidney Transplantation, La Conception Hospital, Aix-Marseille University, APHM, Marseille, France
Christian Saussine, Department of Urology, NHC, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Purpose. To assess the efficacy and safety of intravesical instillation of high molecular weight sodium hyaluronate (HMW- HA) for the treatment of radiation-induced cystitis.
Methods. This prospective cohort study was conducted in seven centers in France. Eligible patients with radiation-induced cystitis were recruited between April 2020 and March 2021. A sterile disposable 50 ml prefilled solution containing 0.16% (80 mg/50 mL) HMW-HA (INSTYLAN) was instilled weekly into the bladder. The treatment consisted of 6 sessions (V1 to V6). Outcomes were assessed 1 week (V7) and 4 weeks (V8) after the last session and were compared with baseline (V0). The primary endpoint was bladder pain, evaluated by a Questionnaire with 5 closed-ended response options. Secondary endpoints included changes from baseline for hematuria, urinary frequency, and the effect of urgencies on Quality of Life (QoL). Adverse events (AEs) were graded according to the CTCAE 3.0 classification.
Results. A total of 30 participants were enrolled. The Intent-to-Treat analysis showed a significant reduction in pelvic pain intensity (− 45.81%, p < 0.001), hematuria (− 26.87%, p = 0.008), total 24 h voids (− 23.92%, p < 0.001) and the effect of urgencies on QoL (− 33.92%, p < 0.001) at V7. The improvement for each outcome remained stable during the post-therapeutic period between V7 and V8. Bladder instillation therapy was well-tolerated: two treatment-related AEs (6.6%) were reported corresponding to two grade 1 hematuria.
Результати:
У дослідження було включено 30 пацієнтів. Аналіз за вибіркою «пацієнти, що мають намір лікуватись» показав суттєве зниження ступеня тазового болю (- 45,81%, р<0.001), гематурії (- 26,87%, p<0.008), добового діурезу (- 23,92%, p<0.001) та впливу позивів на якість життя (- 33,92%, p<0.001) у точці V7. Покращення залишалось стабільним по кожному результату після лікування в період між точками V7 та V8. Лікування внутрішньоміхуровими інстиляціями добре переносилось пацієнтами: повідомлялось про два (6,6%) НЯ, пов’язаних з лікуванням, які відповідали двом випадками гематурії 1 ступеня.
Результаты:
В исследование были включены 30 пациентов. Анализ по выборке «намеренные лечиться» показал существенное снижение в выраженности тазовой боли (- 45,81%, р<0.001), гематурии (- 26,87%, p<0.008), суточном диурезе (- 23,92%, p<0.001) и влиянии позывов на качество жизни (- 33,92%, p<0.001) в точке V7. Улучшение оставалось стабильным по каждому результату после лечения в период между точками V7 и V8. Лечение внутрипузырными инстилляциям хорошо переносилось пациентами: сообщалось о двух (6,6%) НЯ, связанных с лечением, которые соответствовали двум случаям гематурии 1 степени.
Conclusions. Intravesical instillation of HMW-HA appears to be effective in the treatment of radiation-induced cystitis. Further comparative studies with longer follow-up are needed to confirm our preliminary results.
Keywords: sodium hyaluronate, intravesical instillation, radiation-induced cystitis, bladder pain, hematuria.
Introduction
External pelvic radiation therapy is an important tool in the therapeutic arsenal for the treatment of pelvic cancers [1]. However, the bladder is a critical organ that may be sensitive to even low doses of radiation. Despite improved techniques, pelvic irradiation is still responsible for acute and/or late adverse events affecting the bladder. Radiation-induced cystitis is a sterile, noninfectious cystitis characterized by diffuse bleeding from the bladder mucosa. The estimated frequency of radiation-induced cystitis in this exposed population ranges from 5 to 20% [2]. Symptoms include bladder pain, hematuria, increased urinary frequency, urgency, and incontinence, which diminish the affected individuals’ quality of life (QoL), often with further deterioration over time [3].
Treatment of radiation-induced cystitis is challenging. A variety of treatment options are described [4]. Hyperbaric oxygen therapy (HBOT) is the most studied option to treat persistent or recurrent clinically significant hematuria. However, this therapy is expansive, a total of 30 to 40 sessions extending over an 8 week period are needed, and recurrences remain observed [5, 6]. Alternative conservative therapies have been proposed such as intravesical instillations of hyaluronic acid [7]. HA is a component of the glycosaminoglycan layer, found in high concentrations in the sub-epithelial layer of the bladder wall. Intravesical instillation of high molecular weight sodium hyaluronate (HMW-HA): INSTYLAN (Diaco Biofarmaceutici S.R.L., Trieste, Italy) creates a viscoelastic membrane on the mucosal surface, protects, and facilitates regeneration of the damaged urothelium [8, 9].
The aim of the study was to expand evidence on the effect of HMW-HA instillations on symptoms of radiation-induced cystitis.
Materials and methods
Study design and participants
This multicenter prospective cohort study was conducted between April 2020, and March 2021 in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol and amendments were approved by the Committee for the Protection of Persons: CPP Sud-Est VI—Clermont Ferrand. Patients were recruited across seven centers in France.
The Sponsor (LIDDE Therapeutics SAS) and the CRO and Data Management Company (Easy-CRF SAS) were responsible for the overall study management and Data Collection (e-CRF) in collaboration with the Principal Investigator who performed the statistical analysis together with one author. The manuscript was written by one of the authors, independently from LIDDE Therapeutics SAS.
Patients were eligible if they were aged 18 years or older and had late symptoms (> 1 year after radiotherapy) of radiation-induced cystitis caused by pelvic radiation therapy. Inclusion criteria included the presence of bladder pain with at least one other urinary symptoms such as urgency, hematuria, or increased urination frequency.
Previous oral treatment such as anticholinergics was not an exclusion criterion as long as it was discontinued before inclusion. Patients with neurologic bladder, sacral neuromodulation, lower urinary tract infection, post-void residual > 200 mL, clinically significant cardiovascular disease within the past six months, and ongoing treatments including HBOT, glycosaminoglycans replenishment therapy, radiotherapy, brachytherapy or BCG/Mitomycin C instillations were excluded.
Protocol
All patients were seen at the screening visit (V0) to check selection criteria. Patient’s written information and participation agreement was obtained and signed from all patients. Bladder instillations were performed according to the instructions reported in the package leaflet of the manufacturer (Diaco Biofarmaceutici S.R.L., Trieste, Italy).
Briefly, a sterile disposable 50 ml prefilled solution containing 0.16% (80 mg/50 mL) HMW-HA (INSTYLAN) is instilled into the bladder using a sterile 14–16 Foley catheter. Patients were instructed to keep the solution for at least 1 h. The treatment consisted of 6 weekly sessions (V1–V6). No maintenance sessions were scheduled.
Outcomes
Outcomes were assessed by the patient (patient reported outcome measure) and the physician 1 week (V7) and 4 weeks (V8) after the last session and were compared with baseline (V0). The primary endpoint was bladder pain, evaluated by a Questionnaire with 5 closed-ended response options where 1 = no pain, 2 = mild, 3 = moderate, 4 = severe and 5 = unbearable pain.
Secondary endpoints included changes from baseline in other urinary symptoms recorded: hematuria (1 = yellow urine, 2 = pink urine and 3 = red or brown urine), urinary frequency which was evaluated using a 24 h bladder diary, and the effect of urgencies on QoL (1–3 VAS, 1 = no problem/mild, 2 = moderate problem, 3 = severe problem).
Side effects were assessed during weekly visit and were considered separately as adverse events (AEs) and serious adverse events (SAEs). AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 classification.
Statistical analysis
For the sample size calculation, we assumed a 30–50% improvement in bladder pain, a value based on data from a previous study [10]. Using an α = 0.05, we calculated the sample size at n = 16. Considering possible premature trial withdrawals and variations in therapeutic response between studies, we sought to recruit at least 25 patients.
Efficacy and safety analyses were done in the intention-to-treat (ITT) and per-protocol (PP) populations. In the event of patient discontinuation (due to lack of efficacy or side-effects or private reason), the Last Observation Carried Forward (LOCF) imputation method was applied in the ITT analysis. Descriptive statistics were carried out of the available variables. Categorical variables were reported as frequencies and percentages (%), and continuous variables as means and standard deviations (SD). Changes from baseline were assessed using the Wilcoxon signed rank test or the Student’s t test.
The database closure was done (Easy-CRF) on May 20, 2021. Statistical analyses were performed using R Version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). A p value less than 0.05 was considered statistically significant. The study was registered with ClinicalTrials.gov, NCT04696666.
Results
A total of 30 participants were enrolled between April 2020, and March 2021 across seven French centers and formed the ITT population. Excluding three patients who had no bladder pain at V1 and one patient who left the trial after two instillations due to grade 1 hematuria, the PP population comprised 26 patients. Table 1 summarizes the baseline characteristics in the ITT population.
Mean age was 75 years (± 2.8), and 29 (96%) of 30 patients were male. Overall, 87% of patients had bladder pain, 46% had severe hematuria, and 24 h average total voids was 12 (± 1.2). Urgencies had moderate to severe impact on QoL in 87% of included patients. No patient had previous hospitalizations related to hematuria. Radiotherapy was indicated for prostate cancer in 29 cases (96.7%) and cervical cancer in one case (3.3%). The median time from the end of radiotherapy to bladder instillations was 2.3 (2–2.7) years.
Clinical outcomes
A significant reduction in pain intensity was observed at V7 versus baseline in the ITT: 2.27 (± 0.29) (V1) versus 1.23 (± 0.20) (V7) (− 45.81%, p < 0.001) (Table 2). For secondary endpoints, treatment showed significant improvements in hematuria and effects of urgencies on QoL at V7 (all p < 0.01 vs baseline) (Table 2). Diaries demonstrated also significant reduction in total 24 h voids from baseline to V7 [11.87 (± 1.21) to 9.03 (± 1.28)] voids, p < 0.001).
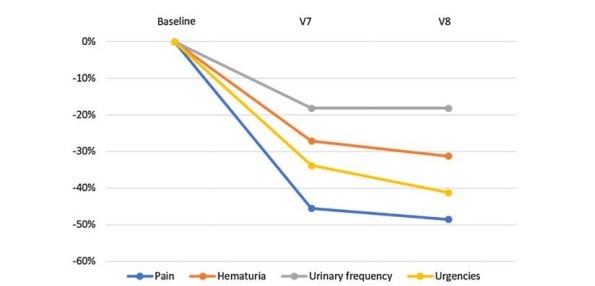
The PP analyses provided similar results: the bladder pain score dropped from an initial mean of 2.46 (± 0.27) (V1) to 1.19 (± 0.18) at the end of treatment (V7) with an average percent change of − 51.62% (p < 0.001). This also applies to the other variables: hematuria (− 34.54%, p < 0.001), number of voids (− 25.17%, p < 0.001) and urgency (− 37.44%, p < 0.001). The improvement for each outcome remained stable and likely slightly higher during the post-therapeutic period (between V7 and V8) (Fig. 1).
Table 1 Baseline characteristics (ITT population)
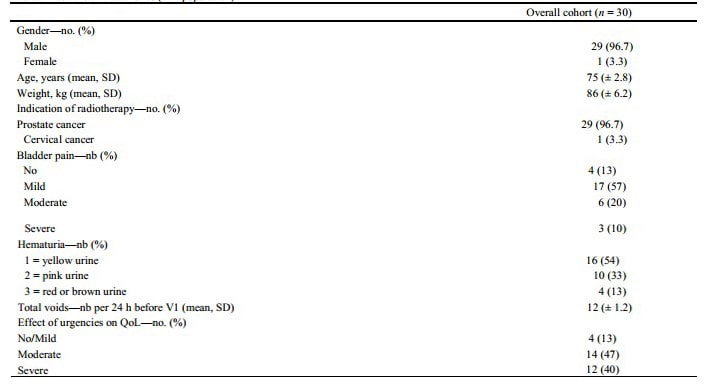
QoL Quality of Life
Safety
Transurethral catheterization and bladder instillation therapy were well tolerated. A total of two treatment-related AEs (6.6%) were reported. They corresponded to two cases of grade 1 hematuria, leading to withdrawal from the study for one patient. No patient required blood transfusion. No serious AEs occurred.
Table 2 Clinical outcomes
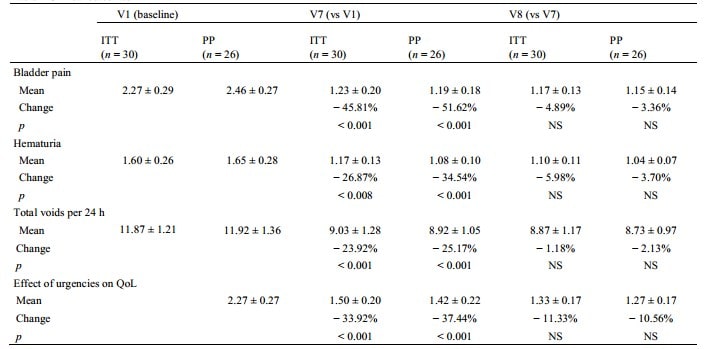
QoL Quality of Life, ITT Intention-to-treat population, PP per-protocol population, NS No significant, V7 one week after the last instillation, V8 four weeks after the last instillation
Fig. 1 Outcomes changes from baseline
Discussion
Our multicenter prospective study supports that intravesical instillation of HMW-HA in patients with radiation-induced cystitis induced by pelvic radiation therapy provides significant clinical benefits. We noted significant improvements in the ITT and PP populations for all outcomes of interest including bladder pain, hematuria, urinary frequency, and the effect of urgencies on QoL. Pelvic radiation therapy has been shown to cause defects in the bladder mucosa, leading to chronic inflammatory changes and delaying or preventing healing of urothelial cells [11].
HMW-HA is a novel intravesical therapy have aimed to replenish the glycosaminoglycan protective layer to reduce exposure of underlying epithelial cells to host urine [12]. Our pilot study confirms the encouraging results obtained in previous studies on HA replenishment therapy in various medical conditions such as interstitial cystitis/painful bladder syndrome [13–15], cystitis induced by intravesical immuno-chemotherapy [16], posthematopoietic stem cell transplantation hemorrhagic cystitis [17], and recurrent bacterial cystitis [18]. The innovative and convincing concept of the HMW-HA instillations therefore prompt its wider use in the management of radiation-induced cystitis.
To date, HBOT is the most studied option to treat persistent or recurrent clinically significant hematuria. Multiple studies have demonstrated that HBOT is safe and effective and should be considered an early treatment option for radiation-induced cystitis in symptomatic patients [19]. HMW- HA has been compared to HBOT in a previous RCT [10]. Patients were randomly assigned to the HA group (n = 16) and the HBOT group (n = 20). At 6, 12 and 18 months after treatment, the improvement rate showed similar outcomes between the two groups. These results strengthen those of our study. The durability of the response observed in our cohort at 4 weeks after the last instillation would thus seem to persist in the medium term. However, longer term follow-up of our cohort is essential to determine how long the action of HMW-HA lasts.
Shao et al. also concluded that intravesical HA was easier to administer and well tolerated than HBOT [10]. Indeed, while HBOT is effective, this treatment has several drawbacks: patients spend 90 min 5–7 days per week in a hyperbaric chamber inspiring 100% oxygen between 2 and 2.4 atmospheres; a total of 40 HBOT treatments extending over an 8‐week period are usually administered [5]; the treatment seems expensive; and its access remains limited to certain expert centers. Thus, due to significant resource and expertise requirements, HBOT use remains limited.
Conversely, we showed that intravesical instillation of HMW-HA is a short treatment, easy to access, requiring no specialized technical platform. In comparison, the extent of the effect of HMW-HA on urinary frequency appears to be similar to antimuscarinic drugs that have been used for many years to treat patients with neurogenic detrusor overactivity [20], showing results promising. Intravesical HA can also have preventive effects. A recently published RCT showed that instillations of hyaluronic acid and chondroitin sulfate given during radiotherapy can prevent urinary symptoms and improve quality of life at 1 year follow-up [21].
Intravesical instillation of HMW-HA was safe and well tolerated. Only two minor AEs 6% (2/30) were recorded. These complications corresponded to two cases of hematuria which were monitored and resolved spontaneously. Our results compare favorably with other intravesical treatments available in radiation-induced cystitis. A recent study used intravesical aluminum salts (alum) in 40 patients with refractory radiation-induced cystitis [22]. Only 13 patients (32.5%) required no additional treatment after a median follow-up of 17 months, bringing the durability of this treatment into question.
In addition, several cases of aluminum toxicity by systemic absorption have been reported in patients with renal failure [23]. Similarly, the use of intravesical formalin, initially reported in 1973, has been associated with frequent severe side effects at the 10% concentration used [24]. Although formalin has been shown to be effective for controlling hematuria, serious complications can occur after treatment, including anuria, hydronephrosis, fistula and septic death [25, 26]. Intravesical instillation of HA seems to solve all of these problems since it does not appear to cause serious AEs.
This pilot study has some potential limitations that should be acknowledged. The main limitations concern its singlearm design and the small number of patients included. Further comparative studies are needed to confirm in a larger cohort the efficacy of INSTYLAN to treat radiation-induced cystitis. The durability of response observed at 4 weeks after the last instillation was encouraging. A 1-year follow-up of our cohort is ongoing to determine how long the action of HMW-HA lasts and whether repeated instillations are necessary. Finally, outcomes were assessed using a visual analogue scale which is not a validated score in this setting.
CONCLUSION
Intravesical instillation of HMW-HA appears to be effective in the treatment of radiation-induced cystitis. Further comparative studies with longer follow-up are needed to confirm our preliminary results.
Author contributions. Study concept and design: MF. Acquisition of data: MF, CC, AC, J-PG, YB, YR, SD, CS. Analysis and interpretation of data: MB, BG-T, and MF. Drafting of the manuscript: MB, MF, and CS. Critical revision of the manuscript for important intellectual content: MF and CS. Statistical analysis: BG-T.
Funding. The study was funded by LIDDE Therapeutics SAS.
Availability of data and materials. Data are available upon request to the corresponding author.
Code availability. None.
Declarations
Conflict of interest. The authors report no conflict of interest.
Ethics approval. The study protocol and amendments were approved by the Committee for the Protection of Persons: CPP Sud-Est VI— Clermont Ferrand.
Consent to participate. Patient’s written information and participation agreement was obtained and signed from all patients.
Consent for publication. All authors approve the submission.
Publisher’s Note. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
LIST OF REFERENCES
- Matz EL, Hsieh MH (2017) Review of advances in uroprotective agents for cyclophosphamide- and ifosfamide-induced hemorrhagic cystitis. Urology 100:16–19
- Payne H, Adamson A, Bahl A et al (2013) Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int 112:885
- Fransson P, Widmark A (1999) Late side effects unchanged 4–8 years after radiotherapy for prostate carcinoma: a comparison with age-matched controls. Cancer 85:678–688
- Mendenhall WM, Henderson RH, Costa JA et al (2015) Hemorrhagic radiation cystitis. Am J Clin Oncol 38(3):331–336
- Ribeiro de Oliveira TM, Carmelo Romao AJ, Gamito Guerreiro FM, Matos Lopes TM (2015) Hyperbaric oxygen therapy for refractory radiation-induced hemorrhagic cystitis. Int J Urol 22:962–966
- Oscarsson N, Müller B, Rosén A et al (2019) Radiation- induced cystitis treated with hyperbaric oxygen therapy (RICH- ART): a randomised, controlled, phase 2–3 trial. Lancet Oncol 20(11):1602–1614
- Zwaans BM, Chancellor MB, Lamb LE (2016) Modeling and treatment of radiation cystitis. Urology 88:14–21
- Browne CD, Davis NF, Mac Craith E et al (2015) A narrative review on the pathophysiology and management for radiation cystitis. Adv Urol 2015:346812
- Pascoe C, Duncan C, Lamb BW et al (2019) Current management of radiation cystitis: a review and practical guide to clinical management. BJU Int 123(4):585–594
- Shao Y, Lu GL, Shen ZJ (2012) Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU Int 109(5):691–694
- Lazzeri ML, Hurle R, Casale P et al (2016) Managing chronic bladder diseases with the administration of exogenous glycosaminoglycans: an update on the evidence. Ther Adv Urol 8:91–99
- Wyndaele JJJ, Riedl C, Taneja R, Lovász S, Ueda T, Cervigni M (2019) GAG replenishment therapy for bladder pain syndrome/ interstitial cystitis. Neurourol Urodyn 38(2):535–544
- Morales A, Emerson L, Nickel JC (1997) Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. Urology 49:111–113
- Shao Y, Shen ZJ, Rui WB, Zhou WL (2010) Intravesical instillation of hyaluronic acid prolonged the effect of bladder hydrodistention in patients with severe interstitial cystitis. Urology 75:547–550
- Ahmad I, Sarath Krishna N, Meddings RN (2008) Sequential hydrodistension and intravesical instillation of hyaluronic acid under general anaesthesia for treatment of refractory interstitial cystitis: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct 19(4):543–546
- Sommariva ML, Sandri SD, Ceriani V (2010) Efficacy of sodium hyaluronate in the management of chemical and radiation cystitis. Minerva Urol Nefrol 62(2):145–150
- Miodosky M, Abdul-Hai A, Tsirigotis P et al (2006) Treatment of post-hematopoietic stem cell transplantation hemorrhagic cystitis with intravesicular sodium hyaluronate. Bone Marrow Transplant 38(7):507–511
- Constantinides C, Manousakas T, Nikolopoulos P, Stanitsas A, Haritopoulos K, Giannopoulos A (2004) Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int 93(9):1262–1266
- Cardinal J, Slade A, McFarland M et al (2018) Scoping review and meta-analysis of hyperbaric oxygen therapy for radiation-induced hemorrhagic cystitis. Curr Urol Rep 19:38
- Madhuvrata P, Singh M, Hasafa Z, Abdel-Fattah M (2012) Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 62(5):816–830
- Redorta JP, Sanguedolce F, Pardo GS et al (2021) Multicentre international study for the prevention with iAluRil of radioinduced cystitis (MISTIC): a randomised controlled study. Eur Urol Open Sci 23(26):45–54
- Westerman ME, Boorjian SA, Linder BJ (2016) Safety and efficacy of intravesical alum for intractable hemorrhagic cystitis: a contemporary evaluation. Int Braz J Urol 42:1144–1149
- Phelps KR, Naylor K, Brien TP et al (1999) Encephalopathy after bladder irrigation with alum: case report and literature review. Am J Med Sci 318:181–185
- Shah BC, Albert DJ (1973) Intravesical instillation of formalin for the management of intractable hematuria. J Urol 110:519–520
- Lojanapiwat B, Sripralakrit S, Soonthornphan S (2002) Intravesicle formalin instillation with a modified technique for controlling haemorrhage secondary to radiation cystitis. Asian J Surg 25:232–235
- Dewan AK, Mohan GM, Ravi R (1993) Intravesical formalin for hemorrhagic cystitis following irradiation of cancer of the cervix. Int J Gynaecol Obstet 42:131–135
Before using the product offered on this information resource, it is recommended to consult your doctor and read the instructions.
Information about medical products for the professional work of health professionals. INSTYLAN (INSTILAN) 0.16% colorless, transparent, viscous gel of hyaluronic acid of non-animal origin, sterile, physiological pH.
INSTYLAN 0,16% approved as Class ІІa medical devices. EU Declaration of Conformity. Registration number: DD 60107286 0001 Manufacturer: Diaco Biofarmaceutici S.R.L. Via Flavia 124, 34147, Trieste, Italy. E-mail: info@diaco.it. www.diaco.it.