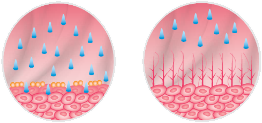
Hyaluronic acid plays a key role in restoring the GAG layer and produces its e ect in the submucosa, exactly where the process of epithelial regeneration initiates.
Sterile Solution based on hyaluronic acid for intravesical irrigation. INSTYLAN is intended for irrigation into bladder cavity that provides the formation of a viscous elastic film on the surface of mucous layer. The irrigation provides temporary protection and restoration of bladder mucous layer during various surgeries, inflammation of the mucous layer of the bladder, radiation therapy.

Hyaluronic acid plays a key role in restoring the GAG layer and produces its e ect in the submucosa, exactly where the process of epithelial regeneration initiates.

Irrigation into the bladder should be provided by a trained medical specialist in specialized premise with appropriate equipment and aseptic conditions.

The study focuses on evaluating the effectiveness of intravesical administration of hyaluronic acid (INSTYLAN) in reducing the frequency of recurrent urinary tract infections (UTIs) and improving symptoms in women. HA helps restore the protective urothelial barrier, reducing bacterial adhesion and inflammation. The study included 24 female patients who received INSTYLAN and 20 women in the control group (fosfomycin). The results showed a significant reduction in the frequency of UTIs in the INSTYLAN group, no serious side effects, and improved patient satisfaction.
Before using the product offered on this information resource, it is recommended to consult your doctor and read the instructions.
Information about medical products for the professional work of health professionals. INSTYLAN (INSTILAN) 0.16% colorless, transparent, viscous gel of hyaluronic acid of non-animal origin, sterile, physiological pH.
INSTYLAN 0,16% approved as Class ІІa medical devices. EU Declaration of Conformity. Registration number: DD 60107286 0001 Manufacturer: Diaco Biofarmaceutici S.R.L. Via Flavia 124, 34147, Trieste, Italy. E-mail: info@diaco.it. www.diaco.it.