SAFETY AND EFFICACY OF INTRAVESICAL ADMINISTRATION FOR SYMPTOM CONTROL IN CHRONIC CYSTOPATHY
Juan Boronat Catalá, Ana Garcia Tello, Laura Gonzalez Montes, Sonia Ruiz Graña, Diego Torres Perez and Louis Llanes Gonzalez
Department of Urology. Getafe University Hospital. Madrid. Spain.
INTRODUCTION: Chronic cystopathy is a widespread pathology. Intravesical hyaluronic acid (instillation) is one of the treatment options for cystopathies. This study analyzed safety and efficacy of intravesical hyaluronic acid instillations in patients with symptoms of urinary blabber disease.
MATERIALS AND METHODS: A population comprising 32 patients received intravesical instillations with hyaluronic acid. Demographic indicators, tolerability and adverse reactions were analyzed, symptoms before and after the treatment were compared. The symptomatic relief achieved as a result of the treatment was assessed with the use of the Patient Global Impression of Improvement (PGI-I) scale.
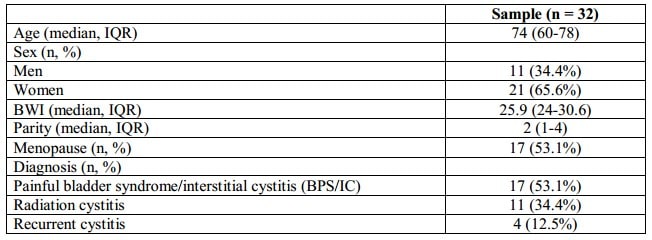
RESULTS: The average age of the patients was 74 years (IQR 60-78), while 65.6% were women. The average observation period was 10 months (IQR 7-14). Eleven patients were diagnosed with radiation cystitis, 17 patients – with painful bladder syndrome/interstitial cystitis (BPS/IC), and 4 patients – with recurrent cystitis. After the treatment, the symptoms improved in 81.8% of patients with radiation cystitis, 82.3% of patients with painful bladder syndrome/interstitial cystitis (BPS/IC), and in 75% of patients with recurrent cystitis.
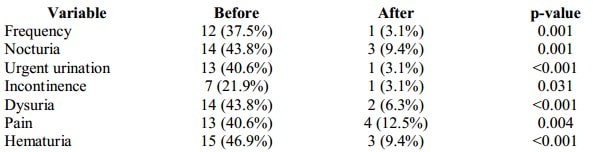
The frequency of hematuria decreased from 46.9% to 9.4% (p<0.001), storage symptoms – from 62.5% to 12.5% (p<0001) and pain sensation – from 40.6% to 12.5% (p=0.004). The treatment was well-tolerated in 100% of patients, and only 2 adverse effects were recorded (urinary tract infection and acute urinary retention). During the observation, 65.6% demonstrated full symptom control and 15.6% – partial symptom control; the highest response was reached in the group of patients with hematuria (73.3%). According to the Patient Global Impression of Improvement (PGI-I) scale, 61.3% of patients experienced the symptomatic relief after the treatment. By the end of the observation period, the symptomatic relief was preserved in 88.9% of patients.
РЕЗУЛЬТАТИ. Середній вік пацієнтів – 74 роки (IQR 60-78), при цьому 65,6% пацієнтів становили жінки. Середній період спостереження – 10 місяців (IQR 7-14). У 11 пацієнтів був діагностований променевий цистит, у 17 пацієнтів – синдром хворобливого сечового міхура/інтерстиціальний цистит (СХСМ/ІЦ), а у 4 пацієнтів – рецидивуючий цистит. Після лікування симптоми покращились у 81,8% пацієнтів з променевим циститом, 82,3% пацієнтів з синдромом хворобливого сечового міхура/інтерстиціальним циститом (СХСМ/ІЦ) та у 75% пацієнтів з рецидивуючим циститом.
Частота гематурії знизилась з 46,9% до 9,4% (p<0,001), симптомів наповнення – з 62,5% до 12,5% (p<0,001), а больових відчуттів – з 40,6% до 12,5% (p=0,004). Лікування добре переносилось 100% пацієнтів. Були зареєстровані лише 2 побічних ефекти (інфекція сечовивідних шляхів та гостра затримка сечі). Під час спостереження у 65,6% пацієнтів було відзначено повний контроль симптомів, у 15,6% пацієнтів – частковий контроль симптомів. Найбільшу відповідь на лікування було досягнуто в групі пацієнтів з гематурією (73,3%). Відповідно до шкали загального враження пацієнта про покращення (PGI-I), 61,3% пацієнтів відчували полегшення симптомів після лікування. Наприкінці періоду спостереження полегшення симптомів зберігалось у 88,9% пацієнтів.
РЕЗУЛЬТАТЫ. Средний возраст пациентов – 74 года (IQR 60-78), при этом 65,6% пациентов составляли женщины. Средний срок наблюдения – 10 месяцев (IQR 7-14). У 11 пациентов был диагностирован лучевой цистит, у 17 пациентов – синдром болезненного мочевого пузыря/интерстициальный цистит (СБМП/ИЦ), а у 4 пациентов – рецидивирующий цистит. После лечения симптомы улучшились у 81,8% пациентов с лучевым циститом, 82,3% пациентов с синдромом болезненного мочевого пузыря/интерстициальным циститом (СБМП/ИЦ) и у 75% пациентов с рецидивирующим циститом.
Частота гематурии снизилась с 46,9% до 9,4% (p<0,001), симптомов наполнения – с 62,5% до 12,5% (p<0,001), а болевых ощущений – с 40,6% до 12,5% (p=0,004). Лечение хорошо переносилось у 100% пациентов; были зарегистрированы только 2 побочных эффекта (инфекция мочевыводящих путей и острая задержка мочи). В ходе наблюдения у 65,6% пациентов был отмечен полный контроль симптомов, у 15,6% – частичный контроль симптомов; наибольший ответ был достигнут в группе пациентов с гематурией (73,3%). Согласно шкале общего впечатления пациента об улучшении (PGI-I), 61,3% пациентов испытывали облегчение симптомов после лечения. К концу периода наблюдения облегчение симптомов сохранилось у 88,9% пациентов.
CONCLUSION: Intravesical hyaluronic acid instillations are a safe and efficient means of treatment for storage symptoms, hematuria and pain syndrome in patients with chronic cystopathies. This treatment option seems to be the most beneficial for patients with radiation-induced cystitis.
Keywords: hyaluronic acid, cystitis, intravesical treatment.
INTRODUCTION
Chronic cystopathies are rampant and present a therapeutic challenge for a urologist. The most widespread conditions include painful bladder syndrome/interstitial cystitis (BPS/IC), recurring cystitis, and radiation-induced hemorrhagic cystitis (or radiation cystitis). The painful bladder syndrome/interstitial cystitis (BPS/IC) is accompanied by bladder-related chronic pain, feeling of pressure or discomfort in the pelvic area that last for more than 6 months and are associated with at least one other symptom of the bladder disease, such as frequent or urgent urination (1).
The estimated prevalence is 10-530 cases per 100,000 population; the significant divergence between the results of the studies is due to different diagnostic criteria and studied populations (2). The recurrent cystitis is defined as 2 episodes of uncomplicated urinary tract infection (UTI) that occur within 6 months or 3 episodes that occur during one year. Around 50% of women experience a UTI in their lifetime, and in 35% of women a UTI recurs within 3-6 months (3, 4). 5-10% of patients who have received radiation therapy experience radiation cystitis; cases of the disease were registered during 20 years after the received treatment (5). All these conditions constitute a significant share of consultations in the urology department, exert a significant impact on the quality of life of the patient and entail a high level of comorbidity.
These cystopathies have a diverse and complex pathophysiology. However, these conditions share one common factor: damaged urothelial glycosaminoglycan (GAG) layer that lines the urinary bladder walls. This defect leads to the loss of physiological protection of the bladder and alters its permeability. The components of urine, such as ions, microorganisms and toxic molecules, penetrate with urothelium and trigger inflammatory processes and local hypersensitivity conditions (6-9).
Hyaluronic acid is a natural proteoglycan, which is part of the extracellular matrix of many tissues and constitutes a significant part of the glycosaminoglycan (GAG) layer on the surface of the urinary bladder. The intravesical use of this substance is assumed to contribute to the restoration of the GAG layer and alleviate the symptoms caused by the layer damage (10). Moreover, hyaluronic acid inhibits the degranulation of labrocytes (mast cells), the activation of which is a critical step in the pathogenesis of interstitial cystitis.
Hyaluronic acid is used in the clinical practice by a urologist for the symptomatic treatment of certain types of cystitis. The purpose of this study is to assess the safety and efficacy of hyaluronic acid in the treatment for these cystopathies.
MATERIALS AND METHODS
A retrospective study was conducted with the participation of 32 patients who received intravesical instillations with hyaluronic acid during the period from September 2018 till January 2020. Patients with bladder cancer, gynecological cancer, urinary lithiasis, active urinary tract infections, pelvic organ prolapse, endometriosis and vaginal candidiasis were excluded from the study. To exclude these pathologies, histories of the present disease were checked, clinical examination, urine culture, cystoscopy, ultrasound scan, flowmetry, and urodynamic study were performed. The necessary tests were selected individually for each patient.
All patients received 4 weekly instillations during the first month, and then from 3 to 6 instillations per month according to the needs of each patient. 0.16% sodium hyaluronate solution (80 mg/50 ml), volume of 50 ml, packed in a sterile easy-to-use package (Instylan®, PreSurgy España SL) was used. The administration was conducted by catheterization of the urinary tract with the use of the indwelling catheter.
Data on the age, parity, previous diseases and surgical interventions, medications taken regularly, symptoms and signs of urological diseases (frequency of daytime and nighttime urination, urgency, incontinence, dysuria, urinary bladder pain, pelvic pain, hematuria), urine culture, admissions to emergency care units, blood transfusion, results of diagnostic visualization and laboratory tests before and after the treatment, as well as adverse events that occurred during instillations, intolerance, adverse reactions, and complications after treatment was collected from the histories of the present disease.
Absence of pain, discomfort or other symptoms during the instillations, which necessitated for immediate discontinuation or conduction of additional instillations, was considered as good tolerability. Full control was defined as the disappearance of all the symptoms studied, and partial control – as preservation of less than 50% of the symptoms after the treatment. The patients assessed their condition according to the Patient Global Impression of Improvement (PGI-I) scale (11), which was used as a retrospective method for assessment of the treatment effect. The scale had a 1 question format; the patients ranged the symptomatic relief associated with the received treatment according to a 7-point Likert scale (Table I).
Statistical analysis
The collected data was analyzed with the use of IBM® Statistics® SPSS 20 software. The categorical variables are presented in absolute values and percentages and were compared using the McNemar’s test. Continuous variables are presented as average values, medians, standard deviation (SD), and range. The normality was checked using the Kolmogorov-Smirnov test. The threshold of statistical significance was set at p<0.05.
RESULTS
The demographic characteristics of the sample are summarized in Table II. 65.6% of the patients included in the study were women with an average age of 74 years (IQR 60-78). The average observation period was 10 months (IQR 7-14). Eleven patients (34.4%) were diagnosed with radiation cystitis, 17 patients (53.1%) – with painful bladder syndrome/interstitial cystitis (BPS/IC), and 4 patients (12.5%) – with recurrent cystitis.
At the baseline, 20 patients (62%) had storage symptoms. 37.5% of all patients presented with frequent urination, 43.8% – nocturia, 40.6% – urgent urination, 21.9% – urine incontinence, and 43.8% – with dysuria. 13 patients (40.6%) demonstrated abdominal pelvic, urethral pain. After the treatment, frequency of storage symptoms decreased from 62.5% to 12.5% (p<0001) and pain symptoms – from 40.6% to 12.5% (p=0.004). Only one patient with recurrent cystitis had new episodes of UTI during the observation period (3 episodes). Table III compares the presence or absence of the studied symptoms before and after the treatment.
Table I. Patient Global Impression of Improvement (PGI I scale)

Table II. Demographic characteristics of the sample
At the baseline, 15 patients (46.9%) presented with hematuria. Of these 15 patients with hematuria, 10 patients (66.7%) were men, 3 patients (20%) underwent treatment with anticoagulants, and 3 patients (20%) received anti-platelet therapy. Of these 15 patients with hematuria, 10 patients (66.7%) underwent radiation therapy (RT) of the small pelvis organs (9 as a result of prostate cancer and 1 as a result of cervical cancer); the average time from radiation therapy to manifestation of the symptoms was 83 months.
Two patients had microscopic hematuria, 5 patients – mild degree macroscopic hematuria, 5 patients – macroscopic hematuria with blood clots, and 3 patients – macroscopic hematuria with anemia, who required the hemostatic transurethral resection (TUR) of the bladder. All patients with radiation-induced hematuria resorted to emergency care for this cause from 1 to 9 times (total of 35 admittances to the emergency department). Of these, 5 patients were hospitalized once or several times and 4 patients underwent blood transfusion due to anemia.
After the treatment, the frequency of hematuria decreased from 46.9% to 9.4% (p<0.001). Only 3 patients resorted again to emergency help, 4 times in total, and none of these patients required hospitalization or blood transfusion.
The treatment was well-tolerated in 100% of patients. Only 2 patients demonstrated adverse effects: urinary tract infection, which required antibiotic therapy, and acute urinary retention, which required bladder catheterization and resolved in 7 days after the catheter removal.
During the observation period, 81.8% of patients with radiation cystitis, 82.3% of patients with painful bladder syndrome/interstitial cystitis (BPS/IC), and 75% of patients with recurrent cystitis demonstrated objective improvement in the symptoms. The Patient Global Impression of Improvement (PGI–I scale) showed that 61.3% of patients experienced symptomatic relief after the treatment, 35.5% did not experience the symptomatic relief, and condition of 3.2% of patients got worse.
Analysis of the answers to this questionnaire with breakdown by the pathologies revealed that 90.9% of patients with radiation cystitis, 47.1% of patients with painful bladder syndrome/interstitial cystitis (BPS/IC) and 25% of patients with recurrent cystitis experienced the symptomatic relief.
65.6% of patients demonstrated full symptom control, and 15.6% – partial symptom control. Full symptom control was achieved in 81.8% of patients with radiation cystitis, in 64.7% of patients with painful bladder syndrome/interstitial cystitis (BPS/IC), and in 25% of patients with recurrent cystitis.
The response to the treatment was different for different symptoms: full control of hematuria symptoms was reached in 73.3% of patients, storage symptoms – in 65% of patients, and pain symptoms – in 53.8% of patients. 88.9% of patients who experienced improvement of the symptoms against the background of the treatment maintained the improvement without changes till the end of the observation period and 11.9% had only temporary improvement, some symptoms re-occurred during the observation period.
Table III. Symptoms of patients before and after instillations
DISCUSSION
Diagnostic assessment and treatment for chronic cystopathies continue to pose a challenge for a practicing urologist. A variety of treatment methods were proposed, most of them were oral and intravesical, but the evidence of proven efficacy of such treatment is insignificant. Our study confirmed that after intravesical instillations with hyaluronic acid, a significant improvement is observed, both in relation to storage symptoms and in relation to pain and hematuria.
Hematuria was a symptom with the best response to the treatment. In our series of tests, most patients with hematuria (66.7%) were diagnosed with radiation cystitis after the exclusion of other possible causes. Several studies have already demonstrated the efficacy of this therapy in radiation hemorrhagic cystitis. In 2011, Shao et al. in a randomized study, which included 36 patients, demonstrated that intravesical hyaluronic acid was as effective as hyperbaric chamber.
A complete response to the treatment was achieved in 87.5% of patients, who received hyaluronic acid (12). That same year, Sommariva et al. observed a significant pain reduction in a study of 69 patients with radiation cystitis (average reduction by 7.7 points according to the Visual Analogue Scale (VAS) for pain assessment after instillations) (13). Recently, Martinez Rodriguez et al. reached the control of hematuria for at least 6 months in 55% of patients in a series of studies that included 20 patients with radiation cystitis (14).
We also observed a significant improvement in the symptoms associated with the interstitial cystitis. The efficacy of intravesical hyaluronic acid in the treatment for interstitial cystitis was extensively studied for many years (2, 10, 15-22). Our findings are very similar to the results of Daha et al., who described an 80% symptomatic relief in a group of 48 patients after 10 weeks of hyaluronic acid instillations (15).
Figueiredo et al. studied 18 patients with painful bladder syndrome and achieved a 50% reduction in the symptoms and a significant improvement in the urodynamic parameters after the treatment (16). Recently, Akbay et al., in a study with the participation of 54 patients with interstitial cystitis, demonstrated a 55% reduction in pain according to the Visual Analogue Scale (VAS) (20). Liang et al. compared symptoms according to the Visual Analogue Scale (VAS) for pain assessment, results of assessment with the use of the Hospital Anxiety and Depression Scale (HADS) before and after treatment with hyaluronic acid in a group of 30 patients with interstitial cystitis versus the control group of 30 healthy volunteers.
Symptomatic relief was observed for urinary tract symptoms (frequency, nocturia, urgent urination and pain) in 73% of patients in 6 months after the treatment, which is similar to the results received in our study (21). Literature sources on the efficacy of hyaluronic acid in the treatment for recurring UTI are scarce, although some authors have already described the reduction in the episodes of the UTI in patients receiving treatment with this substance (2, 8). Our study had lower frequency of cystitis during the observation period; however, the number of patients included with this diagnosis was very insignificant.
Finally, treatment tolerability was excellent (100%) as it was in other studies (2, 10, 15, 16), and only 2 patients had side effects. The results of the PGI-I scale showed that 61.3% of patients experienced symptomatic relief during the treatment, while 48.4% responded that their condition was significantly better or enormously better.
The limitations of our study are its retrospective nature and small volume of the sample, which implies a risk of low statistical power and a lack of comparison with other intravesical agents.
CONCLUSIONS
Intravesical treatment with hyaluronic acid significantly relieves the symptoms in patients with interstitial cystitis and radiation-induced cystitis, is very well tolerated and has very low level of adverse reactions. In our study, patients with radiation-induced cystitis demonstrated a very significant response to treatment, that’s why this treatment method should be considered as an alternative to other treatment methods for this pathology. The studied sample does not enable us to determine the significance of this treatment method in the treatment for recurrent cystitis. To obtain a higher level of evidence, more studies are required, mainly prospective and randomized clinical trials.
LIST OF REFERENCES
- * Van de Merwe J. P., Nordling J., Bouchelouche P., Bushelush K., Cervigni M., Daha L.K. et al. Diagnostic criteria, classification and nomenclature of painful bladder syndrome/interstitial cystitis: an ESSIC proposal. EUR UROL 2008; 53:60-7.
- * Raymond I., Vasdev N., Ferguson J., Haskin M., Davis L., Hasan T.S. The clinical effectiveness of intravesical sodium hyaluronate (Cystistat ®) in patients with interstitial cystitis/painful bladder syndrome and recurrent urinary tract infections. Curr Urol. 2012; 6 (2): 93-8.
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity and economic costs. Am j MED 2002; 113 (Suppl 1A): 5S-13S.
- Foxman B., Gillespie B., Koopman J., Zhang L., Palin K., Tallman P. et al. Risk factors for second urinary tract infection among college women. Am j Epidemiol 2000; 151: 1194-1205.
- Dautruche A., Delouya G. A contemporary review of the treatment for radiation-induced hemorrhagic cystitis. Curr Opin Support Palliat Care 2018; 12:344-350.
- Moldwin R.M., Sant G. Interstitial cystitis: a pathophysiology and treatment update. Clin Obstet Gynecol 2002; 45: 259-72.
- Bassi P., Kostantini E, Foli S., Palea S. Glycosaminoglycan therapy for bladder diseases: emerging new treatments. EUR UROL SUPPL 2011; 10: 451-9.
- Costantinides K., Manousakas T., Nikolopoulos P., Stanitsas A., Haritopoulos K., Giannopoulos A. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU INT 2004; 93: 1262-1266.
- Payne H., Adamson A., Bahl A., Borwell J., Dodds D., Heath C. et al. Chemical- and radiation-induced hemorrhagic cystitis: current treatments and challenges. BJU INT. 2013; 112 (7): 885-97.
- ** Iavazzo C., Athanasiou S., Pitsouni E., Falagas M.E. Hyaluronic acid: an effective alternative treatment of interstitial cystitis, recurrent urinary tract infections and hemorrhagic cystitis? EUR UROL. 2007; 51 (6): 1534-41.
- Yalcin I., Bump R.S. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003; 189: 98-101.
- * Shao Yu., Lu G.L., Shen Z.J. Comparison of intravesical instillation of hyaluronic acid and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU INT 2012; 109: 691-4.
- Sommariva M.L., Sandri S.D., Ceriani V. The efficacy of sodium hyaluronate in the management of chemical and radiation cystitis. Minerva Urol Nefrol 2010; 62: 145- 50.
- ** Martinez Rodriguez R.H., Bayona Arenas S., Ibars Servio L. Hyaluronic acid instillation as treatment of hematuria due to radiation-induced cystopathy. Med Clin (BARC). 2014; 143 (5): 230-1.
- Daha L.K., Riedl C.R., Lazar D., Hohlbrugger G., Pflüger H. Do cystometric findings predict the results of intravesical hyaluronic acid in women with interstitial cystitis? EUR UROL. 2005; 47 (3): 393-7.
- ** Figueiredo A.B., Palma P., Riccetto C., Herrmann V., Dambros M., Capmarthin R. Clinical and urodynamic experience with intravesical hyaluronic acid in painful bladder syndrome associated with interstitial cystitis. ACTAS UROL ESP. 2011; 35 (3): 184-7.
- Morales A., Emerson L., Nickel J.C., Landi M. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol. 1996; 156: 45-8.
- Nordling J., Jorgensen S., Kallestrup E. Cystistat for the treatment of interstitial cystitis: 3-year follow-up study. Urology. 2001; 57.(6 Suppl 1): 123.
- Kallestrup E.B., Jorgensen S.S., Nordling J., Hald T. Treatment of interstitial cystitis with Cystistat®: a hyaluronic acid product. Scand J Urol Nephrol. 2004; 38 (6): 490-4.
- Akbay E, Çayan S, Kılınç C, Bozlu M, Tek M, Efesoy O. The short-term efficacy of intravesical instillation of hyaluronic acid treatment for painful bladder syndrome/interstitial cystitis. Turkish j urol. 2019; 45 (2): 129-34.
- * Liang S.S., Lin Y.H., Hsieh V.K., Huang L. Urinary and psychological outcomes in women with interstitial cystitis/painful bladder syndrome following hyaluronic acid treatment. Taiwan J Obstet Gynecol [Internet]. 2018; 57 (3): 360-3.
- * Sherif H, Sebay A, Kandeel W, Othman T, Fathi A, Mohey A et al. Safety and efficacy of intravesical hyaluronic acid/chondroitin sulfate in the treatment of refractory painful bladder syndrome. Turkish j urol. 2019; 45 (4): 296-301.
* Materials of interest, ** fundamental materials
Before using the product offered on this information resource, it is recommended to consult your doctor and read the instructions.
Information about medical products for the professional work of health professionals. INSTYLAN (INSTILAN) 0.16% colorless, transparent, viscous gel of hyaluronic acid of non-animal origin, sterile, physiological pH.
INSTYLAN 0,16% approved as Class ІІa medical devices. EU Declaration of Conformity. Registration number: DD 60107286 0001 Manufacturer: Diaco Biofarmaceutici S.R.L. Via Flavia 124, 34147, Trieste, Italy. E-mail: info@diaco.it. www.diaco.it.