Intravesical hyaluronic acid instillation in treating recurrent cystitits in women long term results
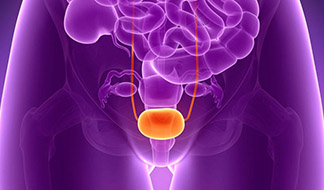
The study focuses on evaluating the effectiveness of intravesical administration of hyaluronic acid (INSTYLAN) in reducing the frequency of recurrent urinary tract infections (UTIs) and improving symptoms in women. HA helps restore the protective urothelial barrier, reducing bacterial adhesion and inflammation. The study included 24 female patients who received INSTYLAN and 20 women in the control group (fosfomycin). The results showed a significant reduction in the frequency of UTIs in the INSTYLAN group, no serious side effects, and improved patient satisfaction.